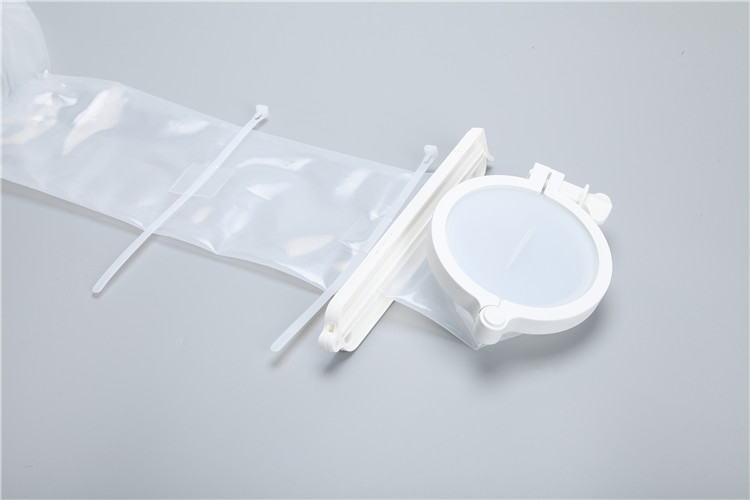
Enteral feeding bags are essential medical devices designed for the safe delivery of nutritionally complete formulas to patients who cannot consume food orally, such as those with dysphagia, critical illness, or gastrointestinal disorders. Unlike other medical fluid containers, they are engineered to maintain the integrity of enteral nutrition (EN) formulas, prevent contamination, and enable controlled (infusion) to support patient metabolism and intestinal function. This article elaborates on the core features, selection criteria, standard operating procedures, troubleshooting methods, and industry trends of enteral feeding bags, providing practical guidance for healthcare professionals and caregivers.
I. Core Features of Enteral Feeding Bags
Enteral
feeding bags are tailored to the unique requirements of EN formula delivery, integrating functional designs that prioritize sterility, formula compatibility, and user-friendly operation. Key features include:
1. Optimized Capacity for Enteral Nutrition Infusion
EN formulas are typically administered in divided doses or continuous infusions over 4-12 hours, so enteral feeding bags have a capacity range of 500mL to 1500mL—aligning with clinical and home care needs. Smaller (500-800mL) bags are suitable for intermittent feeding or patients with limited intestinal tolerance, reducing the risk of formula spoilage during. Larger (1000-1500mL) bags are ideal for continuous infusion in hospitals or long-term care facilities, minimizing the frequency of refilling and reducing contamination risks.
2. Sterile and Anti-Contamination Design
EN formulas are nutrient-rich and prone to bacterial growth, making sterility a top priority. High-quality enteral feeding bags undergo ethylene oxide (EO) or gamma-ray sterilization, with clearly marked sterilization dates and validity periods on the packaging. They feature a sealed, one-way inlet port for adding formulas (equipped with a pierceable membrane compatible with EN formula bottles) and a secure outlet port for connecting infusion tubing—preventing air ingress and external contamination. Some models also include a built-in filter to remove particles from the formula, protecting the enteral catheter from blockage.
3. Controlled Infusion and Flow Regulation
To avoid gastrointestinal discomfort (e.g., bloating, diarrhea) caused by rapid formula delivery, enteral feeding bags are equipped with flow rate regulators (such as roller clamps or dial controls) that allow precise adjustment of infusion speed (ranging from 10 to 120 mL/h). Bags used with enteral pumps feature standardized connectors compatible with pump tubing, ensuring stable and accurate infusion. Additionally, many bags have clear volume graduations (marked in 50mL increments) for easy monitoring of formula consumption and residual volume.
4. Formula Compatibility and Material Safety
The bag material must be compatible with various EN formulas, including those containing proteins, fats, carbohydrates, and acidic/alkaline additives. Common materials include medical-grade polyvinyl chloride (PVC), polypropylene (PP), and silicone: PVC bags are cost-effective and lightweight, suitable for disposable use; PP bags have better chemical resistance and temperature tolerance (-20℃ to 60℃), ideal for formulas that require mild heating; silicone bags are reusable, non-toxic, and resistant to formula adhesion, suitable for long-term home care. All materials must be odorless, non-toxic, and free of substances that could leach into the formula.
5. User-Friendly and Practical Details
Ergonomic designs enhance usability: most bags have a hanging loop or hook for secure placement above the patient (to leverage gravity for infusion), and a transparent or semi-transparent body for visual inspection of formula color and clarity. Some models include a dedicated port for adding medications, avoiding direct mixing with the formula through the main inlet and reducing contamination risks. For light-sensitive formulas (e.g., those containing vitamins B2 or C), opaque or amber-colored bags are available to prevent nutrient degradation.
II. Selection Criteria for Enteral Feeding Bags
Selecting the appropriate enteral feeding bag requires balancing patient needs, clinical safety, formula characteristics, and usage scenarios. The following factors should be prioritized:
1. Compliance with Medical Standards
Qualified enteral feeding bags must meet international standards such as ISO 13485 (medical device quality management system) and local regulatory requirements for enteral nutrition devices. They should be labeled with a medical device registration certificate, production batch number, expiration date, and sterilization method. For patients with allergies, ensure the bag is latex-free and does not contain potential allergens.
2. Capacity and Infusion Mode Matching
Choose the capacity based on the patient’s daily EN requirement and infusion mode: intermittent feeding (3-4 times daily) requires smaller bags (500-800mL) to avoid formula spoilage; continuous infusion (24-hour or 12-hour) benefits from larger bags (1000-1500mL) to reduce refilling frequency. For patients using enteral pumps, select bags compatible with the pump’s tubing system to ensure stable flow control.
3. Material Compatibility with EN Formulas
Consider the formula’s composition when selecting materials: acidic formulas (e.g., those containing fruit extracts) may react with low-quality PVC, so PP or silicone bags are preferred; high-fat formulas require materials with strong oil resistance to prevent leakage. For reusable bags, ensure the material can withstand repeated cleaning and disinfection without degradation.
4. Functional Adaptability to Usage Scenarios
Hospital settings prioritize disposable bags with pump compatibility, filter functions, and medication ports for efficient care. Home care scenarios may require reusable, easy-to-clean bags with clear graduations and simple flow regulators. For patients with mobility needs, lightweight bags with durable hanging loops are more suitable.
III. Standard Usage and Maintenance Procedures
Proper use and maintenance of enteral feeding bags are critical for preventing infections, formula spoilage, and gastrointestinal complications. Follow these standardized procedures:
1. Pre-Use Preparation
- Inspect the packaging: Do not use the bag if the packaging is damaged, damp, or expired, as sterility cannot be guaranteed.
- Practice hand hygiene: Wash hands thoroughly with soap and water or use alcohol-based hand sanitizer before handling the bag and EN formula.
- Check the bag and accessories: For disposable bags, ensure the inlet/outlet ports are sealed, the flow regulator is functional, and there are no cracks. For reusable bags, confirm no residual formula or damage remains from previous use.
- Prepare the formula: Warm refrigerated EN formula to room temperature (avoid heating above 40℃ to preserve nutrients) and shake well before adding to the bag.
2. Formula Loading and Infusion Setup
- Aseptically add the formula: Tear open the bag’s inlet port seal (or pierce the membrane with a sterile syringe) and pour the formula into the bag, avoiding contact between the formula and the port’s outer surface. Do not overfill—leave 50-100mL of space to prevent overflow during infusion.
- Prime the tubing: Connect the enteral tubing to the bag’s outlet port, open the flow regulator, and allow the formula to fill the tubing completely to expel air (air embolism risk is low but can cause patient discomfort).
- Connect to the enteral catheter: Ensure the patient’s enteral catheter (e.g., nasogastric tube, gastrostomy tube) is correctly positioned, then connect the tubing to the catheter securely. For pump-assisted infusion, attach the tubing to the pump and set the desired flow rate.
- Position the bag: Hang the bag 30-45cm above the patient’s stomach to facilitate gravity infusion; ensure the bag is stable and not at risk of falling.
3. During Infusion Monitoring
- Monitor flow rate: Regularly check the infusion speed to ensure it matches the prescribed rate; adjust the regulator or pump settings as needed.
- Observe the patient: Watch for signs of discomfort (e.g., nausea, bloating, abdominal pain) or adverse reactions (e.g., diarrhea, vomiting) and stop infusion immediately if symptoms occur, notifying a healthcare provider.
- Inspect the formula: Check for discoloration, turbidity, or sediment—discard the formula if spoilage is suspected (EN formula should not be infused for more than 4 hours at room temperature).
4. Post-Use Handling and Maintenance
- Dispose of disposable bags: After infusion, empty any residual formula, seal the bag and tubing in a medical waste bag, and discard according to local regulations. Disposable bags should not be reused.
- Clean reusable bags: Rinse the bag and tubing with warm water immediately after use to remove residual formula. Soak in a manufacturer-recommended disinfectant solution (e.g., diluted bleach or enzymatic cleaner) for 30 minutes, then rinse thoroughly with sterile water. Air-dry in a clean, well-ventilated area and store in a sealed container to avoid contamination.
- Replace accessories: Replace reusable tubing every 24-48 hours; replace the bag if cracks, leaks, or material degradation are detected.
IV. Common Problems and Troubleshooting
1. Infusion Flow Interruption
Causes: Tubing kinking, formula sediment blocking the filter or catheter, or insufficient bag height. Solutions: Straighten kinked tubing; flush the catheter with 30mL of warm water (per healthcare provider’s guidance); increase the bag height to 30-45cm; replace the bag if the filter is clogged.
2. Formula Spoilage or Contamination
Causes: Infusion duration exceeding 4 hours at room temperature, non-sterile handling, or damaged bag seals. Prevention: Replace the formula every 4 hours; adhere to aseptic techniques when adding formula; discard bags with damaged seals. If contamination is suspected, stop infusion and monitor the patient for infection symptoms.
3. Patient Gastrointestinal Discomfort
Causes: Rapid infusion speed, cold formula, or formula incompatibility. Solutions: Reduce the flow rate gradually; warm formula to room temperature before infusion; consult a healthcare provider to adjust the formula type or concentration.
4. Leakage at Ports or Connections
Causes: Loose connections, damaged port seals, or overfilled bags. Solutions: Tighten all connections; replace the bag if port seals are damaged; avoid overfilling by leaving sufficient headspace.
V. Industry Trends and Innovations
Driven by the growing demand for home-based enteral nutrition and advancements in patient-centered care, the enteral feeding bag market is evolving with innovative features:
- Intelligent Infusion Monitoring: Emerging models integrate sensors to track formula volume, flow rate, and temperature, transmitting real-time data to healthcare providers via mobile devices. Some smart bags include alarms for flow interruptions or formula spoilage, enabling timely intervention.
- Eco-Friendly and Sustainable Materials: Manufacturers are developing disposable bags made of biodegradable plant-based plastics (e.g., PLA) to reduce medical waste. Reusable bags are being enhanced with antimicrobial materials to extend service life and reduce infection risks.
- Personalized and Specialized Designs: Bags tailored for pediatric patients feature smaller capacities, softer materials, and colorful designs to improve acceptance. High-end models for critical care include integrated temperature control to maintain optimal formula temperature and reduce gastrointestinal discomfort.
- Enhanced Safety Features: Anti-reflux valves prevent formula backflow into the catheter, and tamper-proof seals ensure bag integrity. Some bags include QR codes for traceability, allowing healthcare providers to verify product authenticity and batch information.
VI. Conclusion
Enteral feeding bags play a pivotal role in delivering safe and effective enteral nutrition, directly impacting patient recovery, nutritional status, and quality of life. Selecting a compliant, formula-compatible bag and adhering to standard usage and maintenance procedures are essential for minimizing complications and ensuring optimal care outcomes.
As technology advances, enteral feeding bags will continue to integrate smarter, more sustainable, and patient-centric features. Healthcare professionals and caregivers should stay updated on these innovations to select the most suitable products, tailor care to individual patient needs, and elevate the safety and efficiency of enteral nutrition delivery.