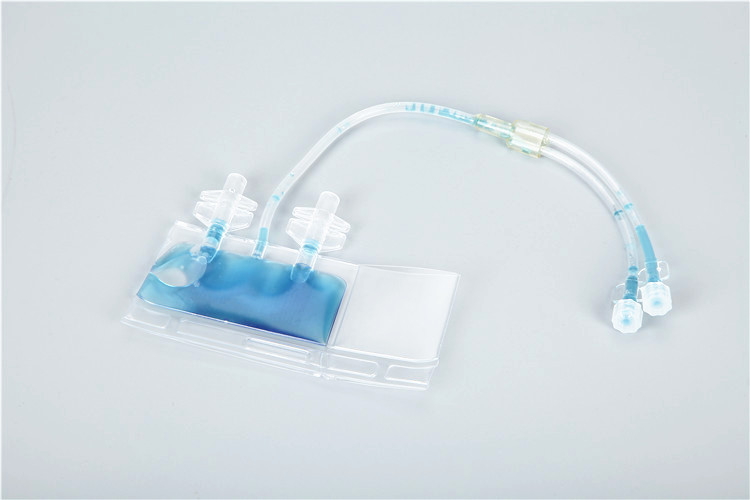
Cryopreservation bags are specialized medical and bioprocessing containers designed for the long-term storage of biological samples at ultra-low temperatures (down to -196℃ in liquid nitrogen). They are widely used in cell therapy, regenerative medicine, biobanking, and pharmaceutical research to preserve the viability and functionality of sensitive biological materials, such as stem cells, blood products, cell lines, and tissues. Unlike traditional cryopreservation vessels (e.g., cryovials), cryopreservation bags offer larger capacity, better flexibility, and reduced risk of sample contamination, making them indispensable for large-scale and clinical-grade sample storage. This article elaborates on the core designs, key types, selection criteria, standard operating procedures, and industry innovations of cryopreservation bags, providing practical guidance for professionals in biomedical and bioprocessing fields.
I. Core Designs and Functional Features of Cryopreservation Bags
Cryopreservation bags are engineered to withstand extreme low temperatures, maintain sample integrity, and prevent contamination—critical requirements for preserving biological materials. Their core designs and features are tailored to address the unique challenges of ultra-low temperature storage:
1. Ultra-Low Temperature Tolerance
The primary requirement for cryopreservation bags is compatibility with ultra-low temperature environments, including vapor-phase nitrogen (-150℃ to -190℃) and liquid nitrogen (-196℃). High-quality bags are made of flexible, low-temperature-resistant materials that remain pliable at extreme cold, avoiding brittleness and rupture. Common materials include polyolefin (PO), ethylene vinyl acetate (EVA), and fluoropolymers—these materials have excellent cryogenic stability, low glass transition temperatures, and minimal shrinkage at ultra-low temperatures, ensuring structural integrity during long-term storage and thawing cycles.
2. Sterile and Contamination-Proof Design
Biological samples are highly susceptible to contamination, so cryopreservation bags undergo rigorous sterilization processes, such as gamma-ray sterilization or ethylene oxide (EO) sterilization, with a sterility assurance level (SAL) of 10⁻⁶. They feature a sealed, tamper-proof design: the inlet/outlet ports (for sample loading and retrieval) are equipped with self-sealing valves or heat-sealable closures to prevent microbial ingress and liquid nitrogen penetration. Disposable cryopreservation bags are pre-sterilized for single use, eliminating cross-contamination risks, while reusable models (rare in clinical settings) are crafted from autoclavable materials with durable sterile barriers.
3. Sample Compatibility and Material Safety
Bag materials must be biocompatible, non-toxic, and free of leachable substances that could damage biological samples or alter their functionality. Polyolefin and EVA are preferred for most applications due to their low protein adsorption, minimal cytotoxicity, and compatibility with cryoprotectants (e.g., dimethyl sulfoxide, DMSO, glycerol)—substances used to prevent ice crystal formation in samples. Fluoropolymers are ideal for sensitive samples (e.g., gene-edited cells, rare cell populations) as they offer superior chemical resistance and reduce sample adhesion. All materials must comply with international standards for biological safety, such as ISO 10993 (biological evaluation of medical devices).
4. Capacity and Handling Convenience
Cryopreservation bags are available in capacities ranging from 1mL (micro-samples) to 500mL (large-scale cell preparations), catering to diverse application needs. Small-volume bags (1-50mL) are suitable for laboratory research, cell line storage, and small-batch clinical samples; large-volume bags (100-500mL) are used for blood bank storage, stem cell therapies, and industrial bioprocessing. Ergonomic designs enhance usability: most bags have a flat or gusseted bottom for stable placement, a transparent body for visual inspection of sample volume and clarity, and a write-on panel or barcode area for sample identification and traceability.
5. Anti-Ice Crystal and Thawing Optimization
To minimize sample damage from ice crystal formation during freezing, some advanced cryopreservation bags feature a multi-layered structure or textured inner surface that promotes uniform heat transfer, facilitating controlled freezing (slow cooling) and reducing ice crystal size. Additionally, the flexible material allows for rapid, uniform thawing when placed in a water bath or thawing device, avoiding thermal gradients that could damage sensitive cells (e.g., stem cells, lymphocytes).
II. Key Types of Cryopreservation Bags by Application
Cryopreservation bags are categorized based on their application scenarios, with specialized designs tailored to clinical, laboratory, and industrial needs:
1. Clinical-Grade Cryopreservation Bags
Used in cell therapy, blood transfusion, and regenerative medicine, these bags prioritize patient safety, traceability, and compliance with clinical regulations (e.g., FDA, EMA). Key types include stem cell cryopreservation bags, blood component bags (for platelets, plasma, red blood cells), and cell therapy bags. They are designed to be compatible with automated cell processing systems, feature barcode labels for full sample traceability, and are manufactured under cGMP (current Good Manufacturing Practices) conditions. Many clinical bags also include a dedicated port for adding cryoprotectants, reducing contamination risks during sample preparation.
2. Laboratory Research Cryopreservation Bags
Widely used in academic and industrial laboratories for cell line storage, molecular biology research, and preclinical studies, these bags emphasize flexibility and cost-effectiveness. Micro-volume bags (1-10mL) are ideal for storing rare cell samples or small batches of cell lines, while medium-volume bags (20-100mL) suit routine laboratory workflows. They are often equipped with easy-to-use closures (e.g., twist-lock valves) and clear volume graduations for precise sample handling. Reusable fluoropolymer bags are occasionally used for long-term storage of valuable cell lines, as they can withstand repeated freezing-thawing cycles.
3. Industrial and Biopharmaceutical Cryopreservation Bags
Designed for large-scale bioprocessing (e.g., bioreactor harvests, bulk cell preparations, vaccine storage), these bags meet strict regulatory requirements for scalability and quality control. Large-capacity bags (200-500mL) with reinforced seams are used for bulk sample storage, while specialized bags with sterile quick-disconnect (SQD) ports facilitate integration into automated bioprocessing lines. They are manufactured with ultra-pure materials to prevent batch-to-batch contamination and feature robust sealing systems to handle high-volume samples during freezing and transportation.
III. Selection Criteria for Cryopreservation Bags
Selecting the appropriate cryopreservation bag requires balancing sample characteristics, storage conditions, regulatory compliance, and application needs. The following factors should be prioritized:
1. Regulatory Compliance and Certification
Qualified cryopreservation bags must meet international standards, including ISO 13485 (quality management system), ISO 10993 (biocompatibility), and ISO 20399 (cryopreservation containers). Clinical-grade bags should obtain Class II or Class III medical device certification (depending on the region) and be labeled with registration numbers, sterilization method, expiration date, and batch information. Industrial bags must comply with cGMP requirements and be validated for sterility, biocompatibility, and cryogenic performance.
2. Material Compatibility with Samples and Cryoprotectants
Match the bag material to the sample type and cryoprotectant used: polyolefin/EVA bags are suitable for most cells, blood products, and DMSO-based cryoprotectants; fluoropolymer bags are preferred for sensitive samples (e.g., gene-edited cells) or harsh cryoprotectants. Ensure the material has low protein adsorption and cytotoxicity to preserve sample viability—avoid materials that may react with cryoprotectants or leach harmful substances.
3. Capacity and Storage Environment
Choose the capacity based on sample volume and storage density: small-volume bags for micro-samples, large-volume bags for bulk preparations. Confirm the bag’s temperature tolerance—ensure it is compatible with the storage environment (liquid nitrogen vs. vapor-phase nitrogen). For liquid nitrogen storage, select bags with reinforced seams and leak-proof closures to prevent nitrogen penetration and sample dilution.
4. Sealing and Retrieval Functionality
Select a sealing method based on application needs: heat-sealable closures offer the most secure seal for long-term storage and clinical use; twist-lock or self-sealing valves are more convenient for laboratory research with frequent sample retrieval. Ensure the inlet/outlet ports are compatible with existing equipment (e.g., pipettes, transfer lines) to facilitate sterile sample loading and retrieval.
5. Traceability and Identification
Prioritize bags with durable write-on panels or barcode-compatible surfaces for sample labeling. Clinical and industrial applications require reliable traceability, so choose bags that can withstand ultra-low temperatures without label fading or barcode degradation. Some advanced models include RFID tags for automated sample tracking in large biobanks.
IV. Standard Usage and Maintenance Procedures
Proper use and handling of cryopreservation bags are critical for preserving sample viability, preventing contamination, and ensuring operational safety. Follow these standardized procedures:
1. Pre-Use Preparation
- Inspect packaging: Do not use bags with damaged, damp, or expired packaging—sterility and cryogenic performance cannot be guaranteed. Check for visible defects (cracks, weak seams, damaged valves) in the bag body.
- Maintain a sterile environment: Operate in a laminar flow hood or biosafety cabinet. Wash hands thoroughly and wear sterile gloves, masks, and gowns to avoid contamination.
- Prepare samples and cryoprotectants: Mix samples with the appropriate cryoprotectant (at room temperature or 4℃, per protocol) to prevent ice crystal formation. Ensure the cryoprotectant is compatible with the bag material.
2. Sample Loading and Sealing
- Aseptically load samples: Use a sterile syringe or transfer line to load the sample into the bag via the inlet port. Do not overfill—leave 10-20% of the bag’s capacity empty to accommodate expansion during freezing (water expands ~9% when frozen).
- Remove air bubbles: Gently squeeze the bag to expel air bubbles, which can cause ice crystal formation and sample damage. For clinical samples, use a needle (if compatible) to aspirate residual air before sealing.
- Securely seal the bag: For heat-sealable bags, use a heat sealer set to the manufacturer’s recommended temperature and time (typically 180-220℃ for 2-5 seconds) to create a tight, leak-proof seal. For twist-lock valves, ensure the valve is fully closed and test for leaks by gently squeezing the bag.
- Label the bag: Immediately label the write-on panel or attach a barcode label with sample information (name, type, collection date, cryoprotectant, storage conditions) using a low-temperature-resistant marker.
3. Freezing and Storage
- Controlled freezing: Use a programmable cryopreservation freezer to cool the bag at a controlled rate (typically -1℃ to -2℃ per minute) to minimize ice crystal damage. Avoid rapid freezing unless the sample protocol specifies otherwise.
- Transfer to storage: Once frozen, transfer the bag to the ultra-low temperature storage system (liquid nitrogen or vapor-phase nitrogen). Ensure the bag is securely placed to avoid puncture or damage from other containers.
- Document storage location: Record the bag’s storage position (rack, drawer, tank) in a sample management system for easy retrieval.
4. Thawing and Sample Retrieval
- Controlled thawing: Remove the bag from storage and thaw it in a 37℃ water bath (or per protocol), gently agitating the bag to ensure uniform thawing. Do not thaw at room temperature or use microwave ovens—this causes rapid ice crystal melting and sample damage.
- Aseptically retrieve samples: Once fully thawed, wipe the bag’s outlet port with 75% ethanol to disinfect. Use a sterile syringe or transfer line to retrieve the sample, avoiding contact between the port and non-sterile surfaces.
- Dispose of or clean the bag: Disposable bags should be sealed in a medical waste bag and discarded according to regulations. Reusable bags (if used) must be thoroughly cleaned with a disinfectant, rinsed with sterile water, and re-sterilized before reuse.
V. Common Problems and Troubleshooting
1. Bag Rupture During Freezing/Storage
Causes: Overfilling (insufficient expansion space), poor material cryogenic stability, puncture from sharp objects, or rapid freezing. Solutions: Leave 10-20% expansion space; select bags with proven ultra-low temperature tolerance; handle bags carefully to avoid puncture; use controlled-rate freezing.
2. Sample Contamination
Causes: Damaged bag packaging, non-sterile handling, improper sealing, or liquid nitrogen contamination. Prevention: Strictly inspect packaging before use; adhere to sterile; ensure a secure seal; use vapor-phase nitrogen storage if liquid nitrogen contamination is a risk. If contamination is suspected, discard the sample.
3. Sample Viability Loss
Causes: Ice crystal formation (rapid freezing), incompatible cryoprotectant, material cytotoxicity, or uneven thawing. Solutions: Use programmable controlled-rate freezing; select a compatible cryoprotectant and bag material; thaw samples uniformly at 37℃; avoid repeated freezing-thawing cycles.
4. Leakage at Seams or Ports
Causes: Improper heat sealing (temperature/time mismatch), damaged valves, or overfilling. Solutions: Adjust heat sealer parameters and test seals before freezing; replace bags with damaged valves; avoid overfilling and ensure proper air removal.
VI. Industry Trends and Innovations
Driven by the growth of cell therapy, biobanking, and personalized medicine, the cryopreservation bag market is evolving with innovative technologies to enhance sample protection, scalability, and traceability:
- Smart Cryopreservation Bags: Emerging models integrate sensors to monitor temperature, pressure, and seal integrity in real time during storage and transportation. Data is transmitted to cloud-based sample management systems, enabling remote monitoring and immediate alerts for anomalies (e.g., thawing, leakage).
- Advanced Material Innovations: Manufacturers are developing new biocompatible materials (e.g., modified polyolefins, nanocomposite polymers) with reduced protein adsorption, enhanced cryogenic stability, and improved resistance to cryoprotectants. Antimicrobial coatings are also being added to prevent microbial growth during sample handling.
- Automation-Compatible Designs: Bags with standardized sterile connectors and RFID tags are being developed to integrate with automated sample processing and storage systems, reducing human error and improving workflow efficiency in large biobanks and cell therapy facilities.
- Single-Use and Sustainable Solutions: The shift toward single-use systems in bioprocessing is driving demand for cost-effective, disposable cryopreservation bags. Manufacturers are also developing eco-friendly materials (e.g., biodegradable polyolefins) to reduce environmental impact without compromising performance.
- Customized Clinical-Grade Bags: For personalized cell therapies, customized bags with patient-specific traceability features (e.g., embedded RFID chips) and specialized capacities are being developed to meet strict clinical regulations and ensure sample safety.
VII. Conclusion
Cryopreservation bags are critical components in the storage and transportation of sensitive biological samples, directly impacting sample viability, research reproducibility, and clinical therapy outcomes. Selecting compliant, sample-compatible bags and adhering to standard usage procedures are essential for minimizing risks, preserving sample integrity, and meeting regulatory requirements.
As technology advances, cryopreservation bags will continue to evolve with smarter, more sustainable, and application-specific features. Professionals in biomedical research, cell therapy, and bioprocessing should stay updated on these innovations to select the most suitable products, optimize sample storage workflows, and support the growing demands of personalized medicine and regenerative therapy.